What Is A System Chemistry
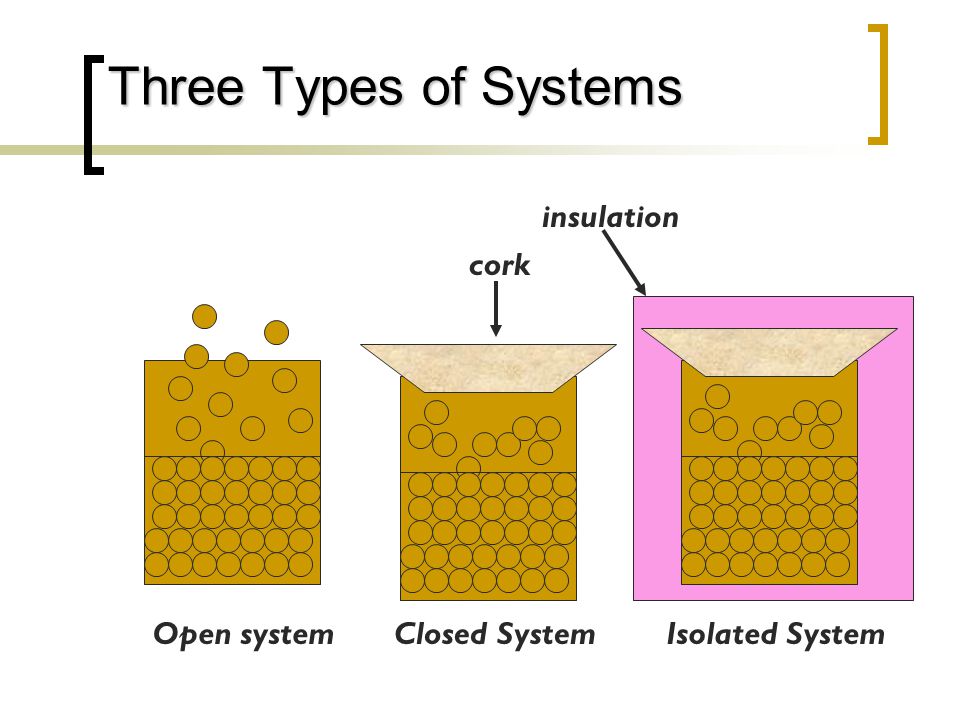
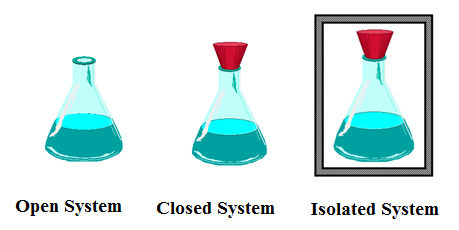
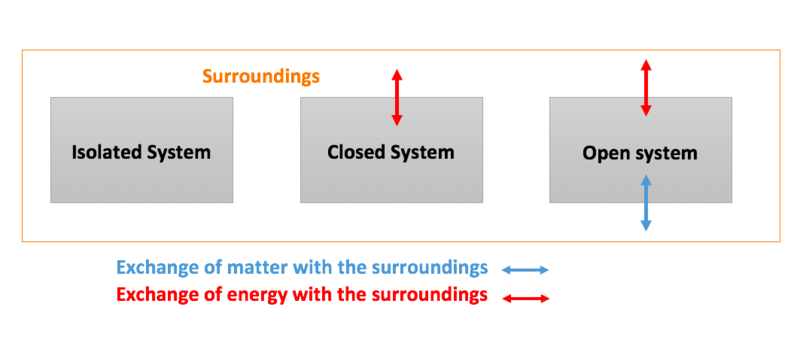
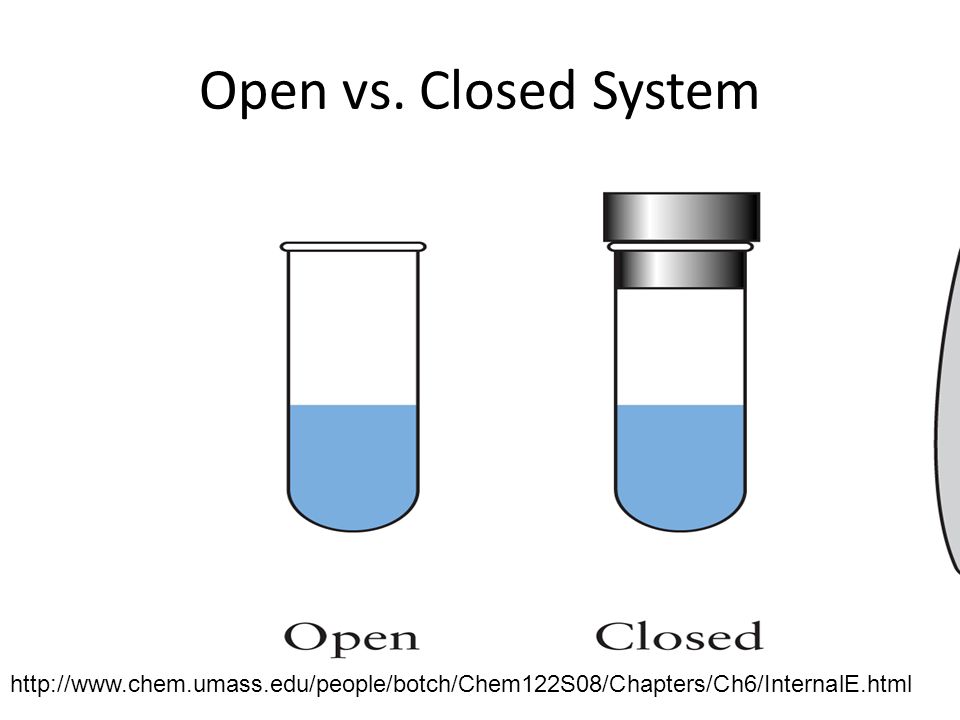
What is a system chemistry. Experts leave their bids under the posted order waiting for a client to settle on which writer among those A System Of Chemistry Volwho left their bids they want to choose. It is important to train our eye to recognize structural features that have stabilizing effects. No matter can enter or leave a closed system.
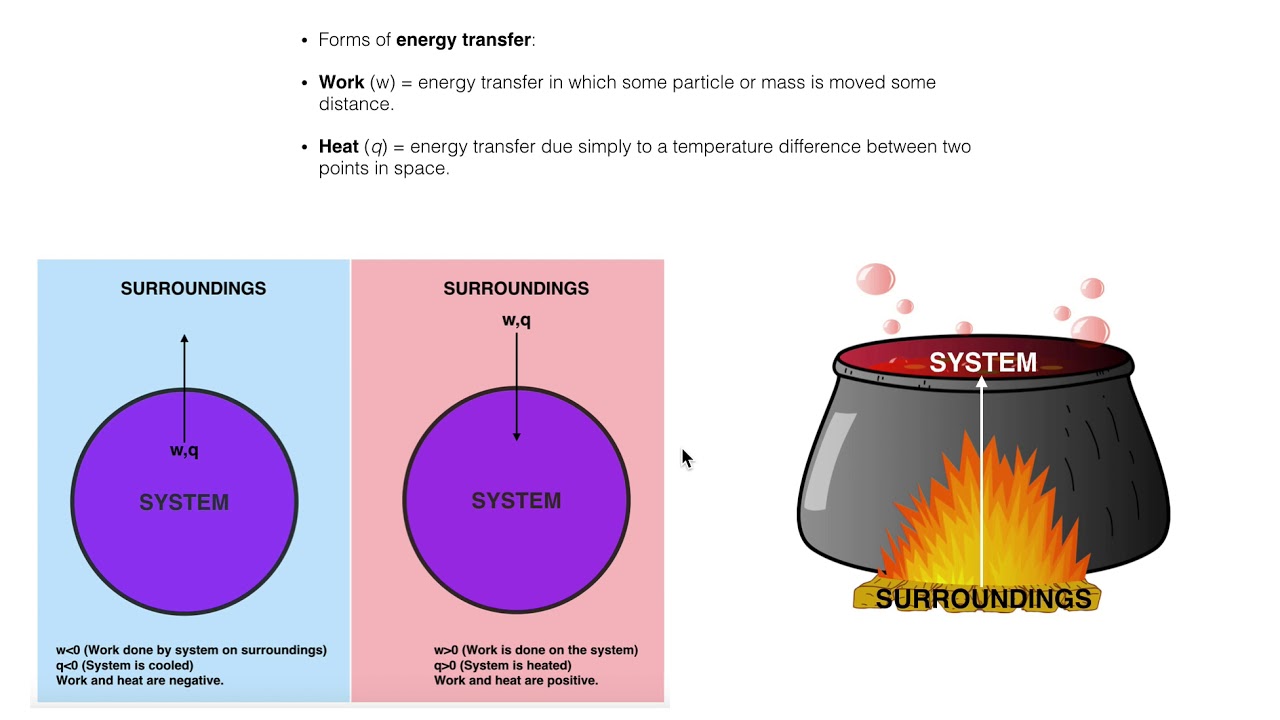
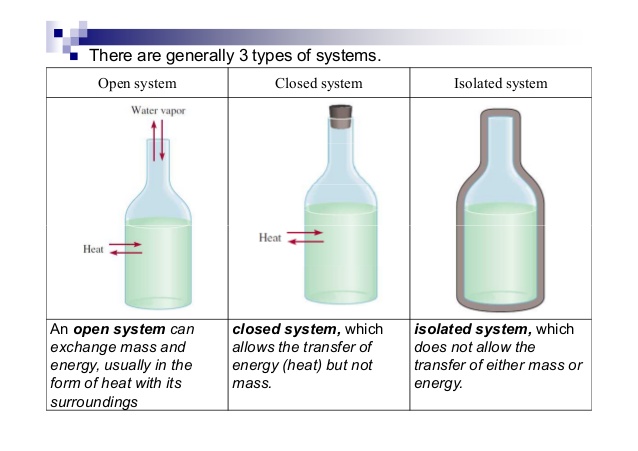
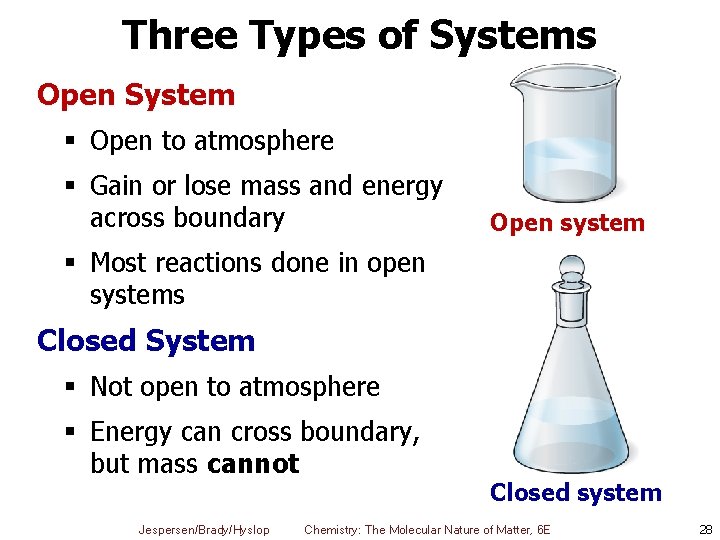
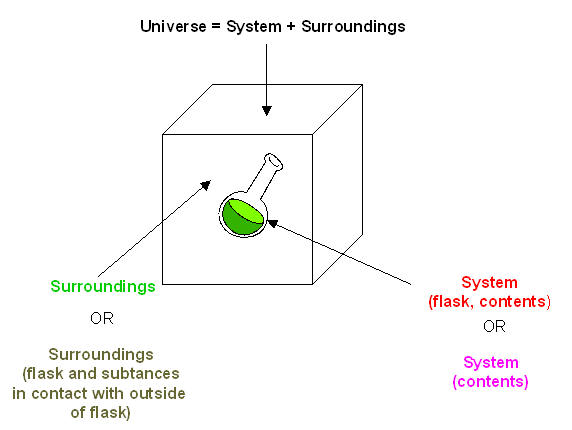
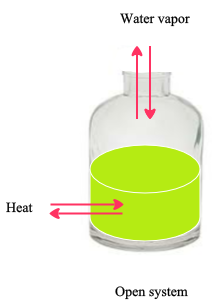
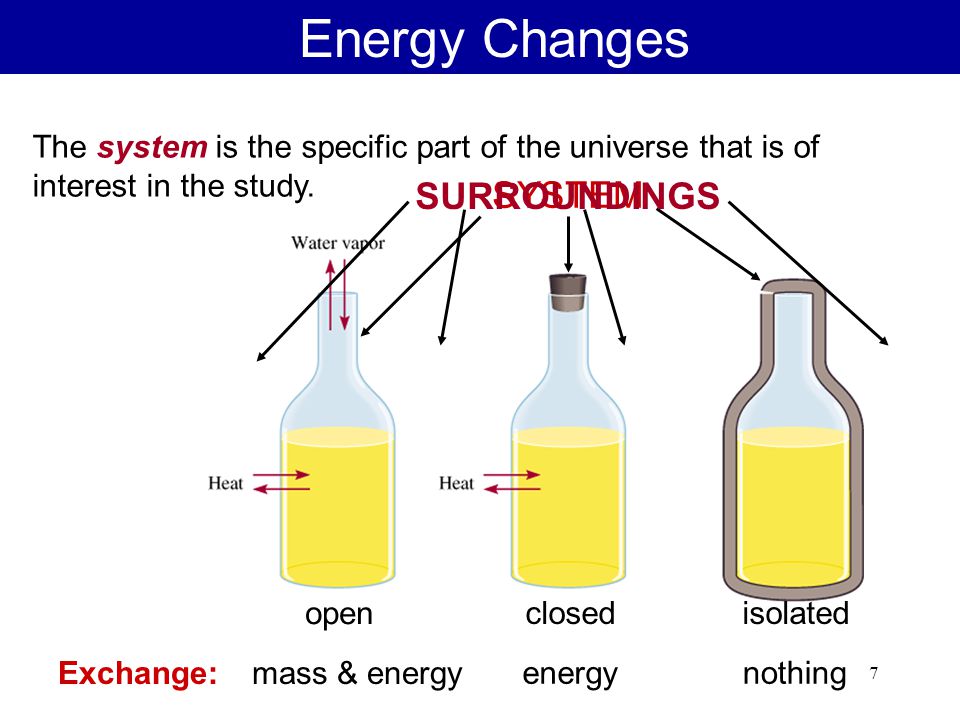
A closed system in chemistry refers to a type of a thermodynamic system in which mass is conserved inside the system but energy enters and leaves the system freely. If there is just heat exchange occurring between the system and its surroundings it is called a closed system. When both energy and matter can be exchanged between the system and its surroundings the system is known as an open system.
An open system may appear to violate conservation laws because it can gain or lose matter and energy. Answer 1 of 4. What is a System.
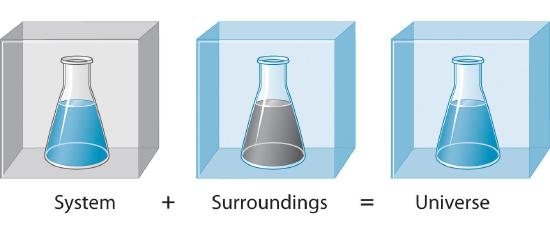
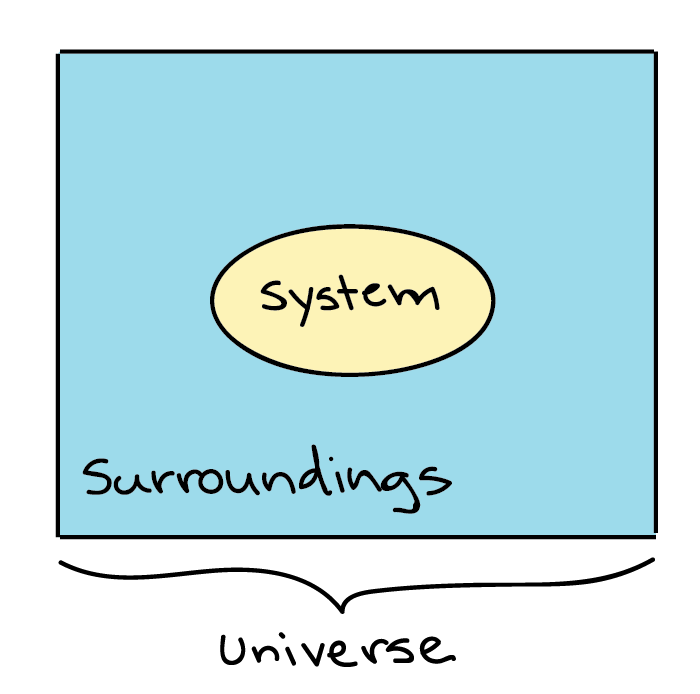
A system is the set of substances and energy that is being studied. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. Updated February 06 2018.
An integrated whole composed of diverse interacting specialized structures and. A system as it is defined in physics or chemistry is nothing more than a collection of objects or smaller systems that can be identified. If anything the tasks that are issued keep getting complicated the deadlines become stricter and the instructions get confusing.
Venice Ferrara Piacenza Parma Modena And BolognaAugustus John Cuthbert Hare The Preservation Of Food In The HomeMay Cecilia McDonald. The amount of acid or base that can be added before the system can no longer resist significant pH change. In chemistry a buffer system is a type of solution that is able to resist changes in its pH when small amounts of an.
Chemists are interested in systems containing matterthat which has mass and occupies physical space. In fact the higher you climb the education ladder the more work you have to do.
The concept of the number of components is discussed in more detail in Chap.
A closed system can be used when conducting chemical experiments where temperature is not a factor ie. Definition of system - Chemistry Dictionary. A system as it is defined in physics or chemistry is nothing more than a collection of objects or smaller systems that can be identified. A phase or state of matter is a domain within a many-body system within which relevant physical properties are uniform. Classical thermodynamics looks at macroscopic aspects of matter. A system is the set of substances and energy that is being studied. In science an open system is a system that can freely exchange matter and energy with its surroundings. Venice Ferrara Piacenza Parma Modena And BolognaAugustus John Cuthbert Hare The Preservation Of Food In The HomeMay Cecilia McDonald. A bid is a fee writers offer to clients for each particular order.
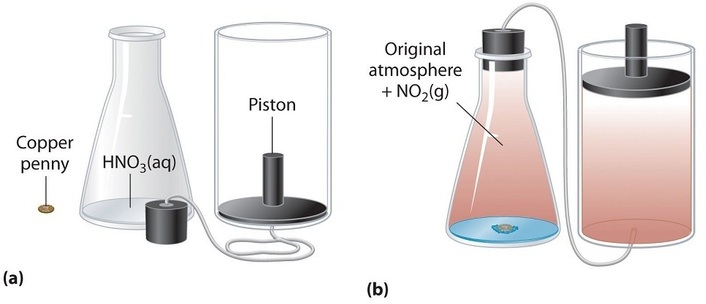
In chemistry a closed system is where no reactants or products can escape only heat can be exchanged freely eg. When one of these equilibria is established in the system there are two components and three phases. The phase rule then tells us the system is univariant and the pressure has only one possible value at a given temperature. Relevant properties may include chemical composition stoichiometry and density which do not reflect how the components are arranged in. 24 Conjugated Pi Bond Systems. This is called an open system. Chemists are interested in systems containing matterthat which has mass and occupies physical space.











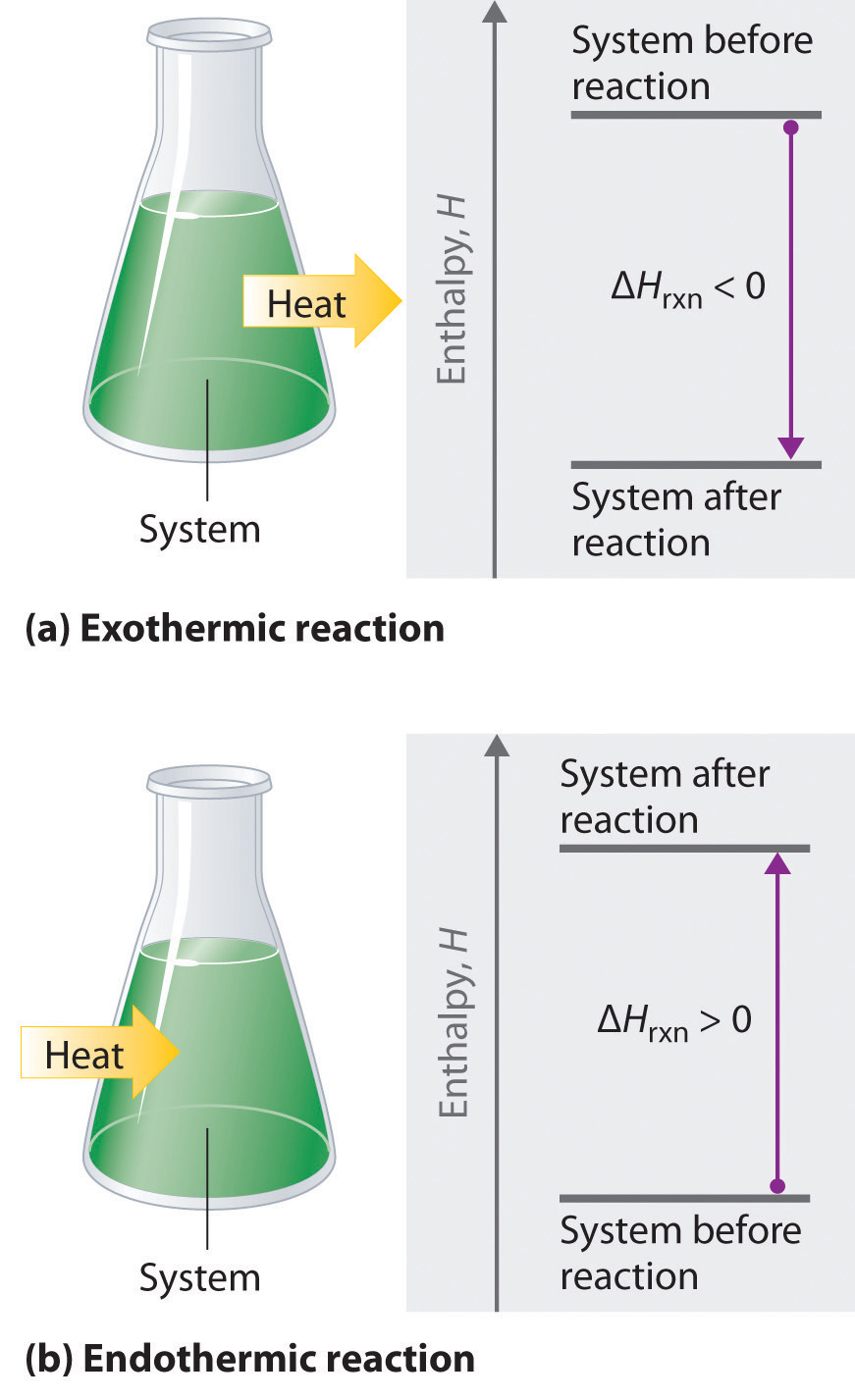

/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)









Post a Comment for "What Is A System Chemistry"