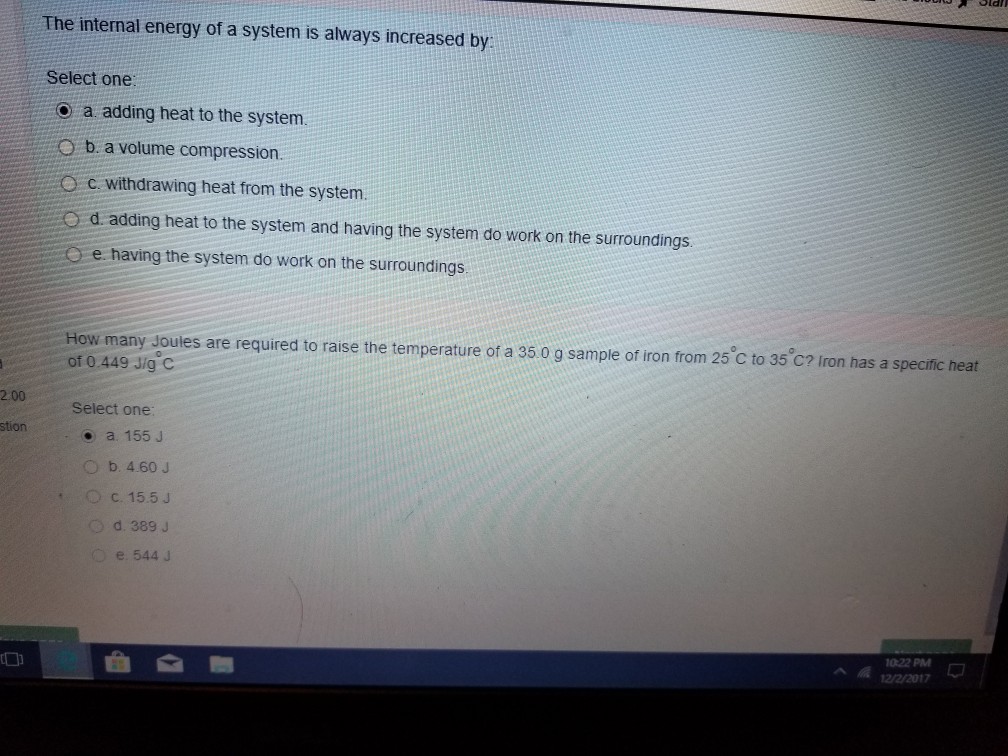
The Internal Energy Of A System Is Always Increased By
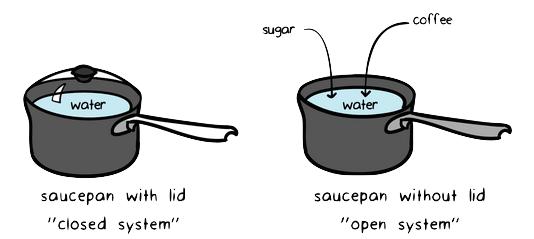
The internal energy of a system is always increased by. This system is thermally isolated from its surroundings. In open systems the mass that crosses the boundary between the surroundings and the system always contributes to two. How much work was done.
When energy is given to raise the temperature. What is ΔV if pressure is constant at 1 atm. This tells us the following.
So if I said I did all of this stuff and came back here what is my. The internal energy is the total amount of kinetic energy. More_vert Does adding heat to a system always increase its internal energy.
Application of the thermal energy often called the internal energy of systems. The internal energy of a system increased by 982 J when it absorbed 492 J of heat. Details of the calculation.
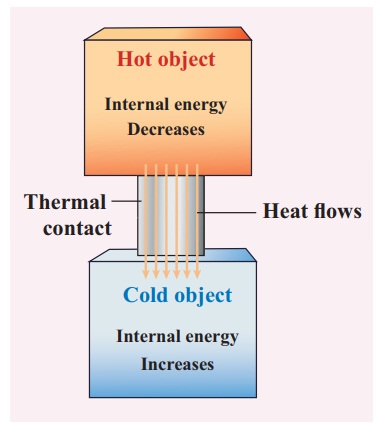
Any heat interaction that takes place in the system with its surroundings also changes its internal energy. Was work done by or on the system. Physics University Physics Volume 2 Does adding heat to a system always increase its internal energy.
So the internal energy is always going to be the same at this point. A thermodynamic system undergoes a process in which its internal energy decreases by 500 J. The second law states that entropy of the universe always increases.
One of the central concepts of thermodynamics is temperature. The entropy of the combined system has increased.
The internal energy per unit mass of the system is the specific internal energy u.
The system gained 90 kJ of energy as heat. A thermodynamic system undergoes a process in which its internal energy decreases by 500 J. The system gained 210 kJ of energy as heat. The internal energy would increase if work is done on the system and decreases if work is done by the system. The second law states that entropy of the universe always increases. The internal energy per unit mass of the system is the specific internal energy u. The internal energy is the total amount of kinetic energy. When energy is given to raise the temperature. Was work done by or on the system.
The system lost 90 kJ of energy as heat. The system lost 210 kJ of energy as heat. The internal energy of a system of constant composition can be changed by work or heat interactions with its surroundings. Instead it is caused by electrical energy crossing the system boundary and thus. Increase in internal energy of a system heat put into the system - work done by the system on its surroundings. We learned that for a particular system there will always be a conservation of energy. The internal energy of a system can be increased by increasing the heat transfer but because of factors such as surface deformation friction there may be some energy loss.



































Post a Comment for "The Internal Energy Of A System Is Always Increased By"